For copper electrowinning (EW) plant managers, the anode is more than just a component; it’s the foundation of your entire operation. Its performance directly impacts energy consumption, cathode copper purity, operational uptime, and your bottom line. While the basic concept of a lead alloy anode is simple, the devil is truly in the design details.
At PRS, we believe that superior performance is engineered from the start. It’s the meticulous attention to these critical design elements that separates a standard anode from a high-performance workhorse. Here are the five non-negotiable design details we prioritize to ensure our anodes deliver maximum reliability and efficiency.
1. Precision Alloying: The Metallurgical Foundation
The choice of alloying elements and their precise ratios is the first and most critical decision. A simple lead plate would quickly fail under the harsh conditions of an EW cell. We use a multi-element Pb-Ca-Sn (Lead-Calcium-Tin) alloy system, often enhanced with other elements like Strontium.
Why it matters: Calcium and Tin promote the formation of a hard, dense, and highly conductive lead dioxide (PbO₂) layer during operation. This layer is essential for the oxygen evolution reaction and, crucially, protects the underlying lead from excessive corrosion.
Our approach: Our proprietary alloy formulation is optimized for uniform distribution of these elements, ensuring consistent electrochemical behavior across the entire anode surface. This uniformity prevents localized corrosion, extends service life beyond 4-5 years, and contributes to stable cell voltage for lower energy costs.
Basis for experimental and project operational results:
1. Measured results of anode potential: As the tin content increases, the anode potential gradually decreases. This is because tin can reduce the generation of oxygen ions and promote the formation of PbO2. In addition, the dissolution of tin increases the porosity of the anode film, inhibiting the formation of high-impedance PbO and PbSO4 on the anode surface, thus promoting the formation of PbO2. When the tin content further increases to 1.4%, the anode potential is almost the same as that with a tin content of 1.2%.
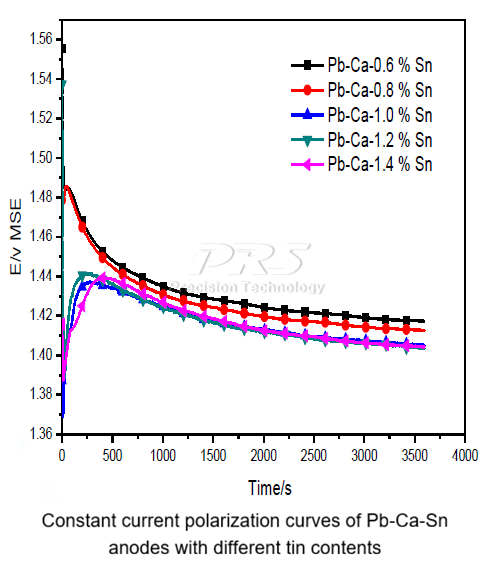
2. Measured results of cell voltage: The Pb-Ca-1.2%Sn anode has the lowest cell voltage, while the Pb-Ca-1.4%Sn anode has a relatively lower anode overpotential, and the Pb-Ca-1.2%Sn anode exhibits better reversibility.
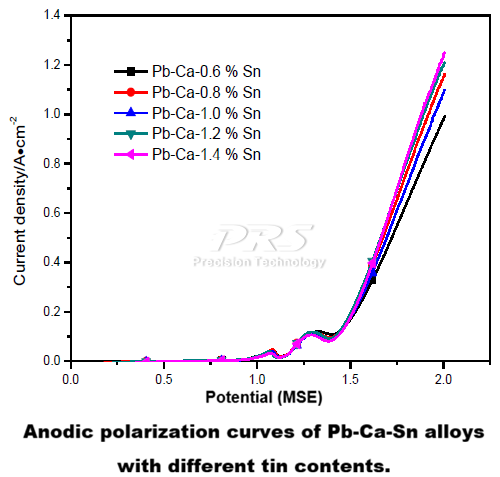
| Anode | a/v | b/v | i (A·cm-2) |
| Pb-Ca-0.6%Sn | 1.3521 | 0.6615 | 0.904×10-2 |
| Pb-Ca-0.8%Sn | 1.30 | 0.6451 | 0.966×10-2 |
| Pb-Ca-1.0%Sn | 1.3196 | 0.6414 | 0.876×10-2 |
| Pb-Ca-1.2%Sn | 1.2483 | 0.6396 | 1.12×10-2 |
| Pb-Ca-1.4%Sn | 1.2758 | 0.5986 | 0.739×10-2 |
Conclusion: Therefore, controlling the tin content in the alloy between 1.2% and 1.4% is considered reasonable.
We recommend the following reasonable alloy composition: Ca: 0.06–0.08%, Sr: 0.04–0.05%, Sn: 1.2–1.4%, with the remainder being lead.
2. Robust Joint Integrity: The Trapezoidal Bayonet Weld
A lead alloy plate (or blade) is heavy. Over years of service, the stress on the weld connecting the blade to the conductive copper beam can be immense. A weak joint is a primary point of failure. Our solution is a patented Trapezoidal Bayonet seamless welding technique.
Why it matters: This design creates a massive mechanical interlock between the blade and the copper beam beforewelding. The trapezoidal shape prevents any lateral or vertical movement. The subsequent seamless welding then creates a complete metallurgical bond.
The benefit: This joint is exceptionally strong, virtually eliminating the risk of separation or “bulging” during operation. It ensures optimal electrical conductivity, minimizes voltage drop, and guarantees the structural integrity of the anode for its entire lifespan.


3. Optimized Hydrodynamics: Strategic Hole Placement for Insulators and Flow
The design isn’t just about the metal; it’s about managing the electrolyte. A common standard is to install insulators at at least three points: both bottom ends and the center. However, we recommend going a step further by adding more holes.
Why it matters: The primary function of the holes is to accommodate insulator pins that prevent short-circuiting with the cathode. But additional, strategically placed holes significantly enhance electrolyte circulation.
The benefit: Improved copper ion mobility ensures a more consistent concentration at the cathode surface, promoting even copper deposition and reducing the risk of nodules or dendrites. This enhanced flow also helps dissipate heat and oxygen bubbles, contributing to a smoother, more efficient process and higher-quality cathode copper.

4. Dimensional Precision: The Anode-to-Cathode Ratio
The physical dimensions of the anode relative to the cathode are not arbitrary. To produce the high-grade, smooth-edged cathode copper demanded by the market, the anode must be correctly sized. Our standard design calls for a lead anode blade that is 30mm narrower in width and 35-40mm shorter in height than the stainless steel cathode plate.
Why it matters: This specific dimensional difference creates a controlled current density distribution at the edges of the cathode. If the anode is too large, the electrical field will cause excessive copper deposition on the edges of the cathode sheet, creating rough, uneven “ears” that are problematic in handling and packaging.
The benefit: You achieve a perfectly flat, dense cathode copper plate with smooth edges, ready for the market without additional processing. This directly translates to a premium product that meets LME Grade A standards.

5. Protecting the Critical Zone: The Liquid Line-to-Weld Distance
The area of the anode around the electrolyte liquid line is subjected to a particularly harsh environment of alternating immersion and exposure, leading to accelerated corrosion. A key design parameter is the distance from the top weld (connecting the blade to the hanger bar) to the operating liquid line.
Why it matters: We strictly maintain this distance at 150mm ±50mm. Keeping the weld safely above the corrosive splash zone and the area of greatest oxidative stress is vital.
The benefit: This design significantly reduces the risk of corrosion attacking the critical weld joint, preserving the anode’s structural and electrical integrity for years. It’s a simple specification that has a profound impact on long-term durability.

Engineering Excellence is in the Details
At PRS, we don’t just manufacture anodes; we engineer them for peak performance. Each of these design details is backed by decades of metallurgical science and hands-on experience in copper electrowinning plants worldwide.
By focusing on these critical elements, we ensure our anodes provide:
- Longer Service Life: Reducing operational downtime and replacement costs.
- Lower Operating Costs: Through optimized voltage and energy efficiency.
- Higher Cathode Purity: Minimizing lead contamination and producing A-grade copper.
- Higher Reliability: Giving you peace of mind and predictable production.
Ready to Experience the Difference that Precision Design Makes?
If your operation is ready to benefit from anodes built with this level of expertise, we are here to help. Our engineering team is ready to discuss your specific cellhouse conditions and provide a customized solution.

